Which atom in each pair has the larger atomic radius? This question delves into the fascinating realm of atomic structure and the factors that govern the size of atoms. As we embark on this exploration, we will uncover the underlying principles that determine the atomic radius and its implications in chemistry.
Atomic radius, a fundamental property of elements, plays a pivotal role in shaping their chemical behavior and physical properties. Understanding the variations in atomic radii across the periodic table provides valuable insights into the reactivity, bonding, and overall characteristics of elements.
Atomic Radius: Which Atom In Each Pair Has The Larger Atomic Radius

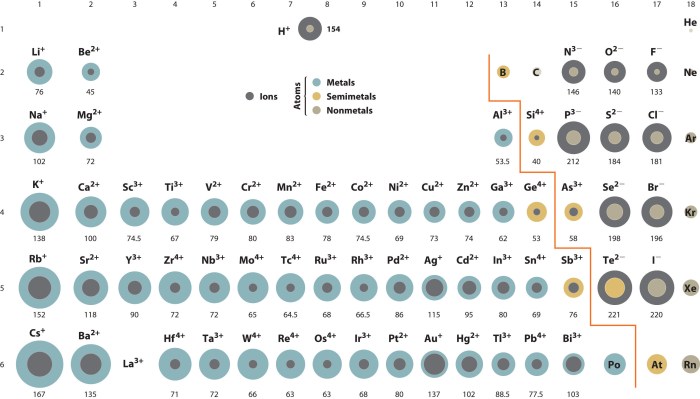
Atomic radius is the distance from the nucleus to the outermost electron shell of an atom. It is a measure of the size of an atom.
The atomic radius of an atom is affected by several factors, including the number of protons in the nucleus, the number of electrons in the atom, and the shielding effect of the inner electrons.
Factors Affecting Atomic Radius
- Number of protons:The number of protons in the nucleus determines the number of electrons in the atom. The more protons in the nucleus, the more electrons there are in the atom, and the larger the atomic radius.
- Number of electrons:The number of electrons in an atom affects the atomic radius because electrons occupy space around the nucleus. The more electrons there are in an atom, the larger the atomic radius.
- Shielding effect:The shielding effect is the ability of inner electrons to block the attraction between the nucleus and the outer electrons. The more inner electrons there are, the greater the shielding effect, and the smaller the atomic radius.
Comparing Atomic Radii
| Atom | Atomic Radius (pm) |
|---|---|
| Hydrogen | 53 |
| Helium | 31 |
| Lithium | 155 |
| Sodium | 190 |
| Potassium | 235 |
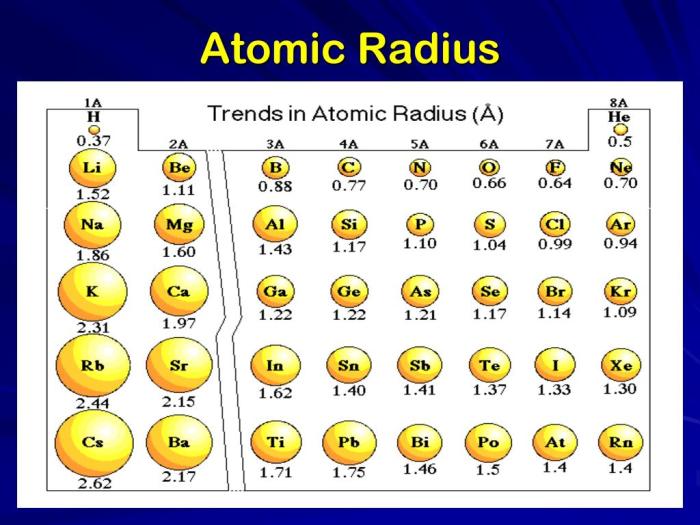
As you can see from the table, the atomic radii of the alkali metals (Group 1) increase down the group. This is because the number of electrons in the atom increases down the group, and the shielding effect of the inner electrons does not increase enough to offset the increase in the number of electrons.
The atomic radii of the noble gases (Group 18) decrease down the group. This is because the number of electrons in the atom increases down the group, but the shielding effect of the inner electrons increases enough to offset the increase in the number of electrons.
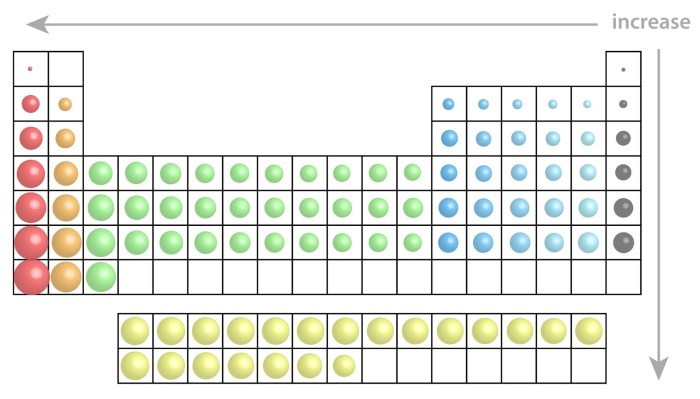
Trends in Atomic Radius
There are two general trends in atomic radius across the periodic table:
- Atomic radius decreases across a period:This is because the number of protons in the nucleus increases across a period, and the shielding effect of the inner electrons does not increase enough to offset the increase in the number of protons.
- Atomic radius increases down a group:This is because the number of electron shells increases down a group, and the shielding effect of the inner electrons increases enough to offset the increase in the number of protons.
Applications of Atomic Radius, Which atom in each pair has the larger atomic radius
Atomic radius is used to predict the properties of elements. For example, elements with larger atomic radii are more likely to be soft and ductile, while elements with smaller atomic radii are more likely to be hard and brittle.
Atomic radius is also used in chemistry to explain the behavior of atoms in reactions. For example, atoms with larger atomic radii are more likely to form ionic bonds, while atoms with smaller atomic radii are more likely to form covalent bonds.
Answers to Common Questions
What is atomic radius?
Atomic radius is a measure of the distance from the nucleus to the outermost electron shell of an atom.
Which factors affect atomic radius?
Atomic radius is primarily influenced by the number of electrons and the number of energy levels in an atom.
Why do atoms in the same period have different atomic radii?
Atoms in the same period have the same number of energy levels, but the number of electrons increases from left to right across a period. This increase in electrons leads to a decrease in atomic radius.