Predict the product for the following diels-alder reaction – Delving into the intricacies of predicting the product for the following Diels-Alder reaction, this introduction immerses readers in a unique and compelling narrative, with authoritative tone that is both engaging and thought-provoking from the very first sentence. The Diels-Alder reaction, a cornerstone of organic chemistry, offers a powerful tool for constructing complex molecular architectures, and understanding its intricacies is crucial for harnessing its full potential.
This comprehensive guide will provide a detailed exploration of the Diels-Alder reaction mechanism, enabling readers to decipher the factors governing product formation and optimize reaction conditions for desired outcomes. Through a blend of theoretical insights and practical examples, we will unravel the intricacies of this versatile reaction, empowering readers to confidently predict products and harness its synthetic prowess.
Reaction Mechanism: Predict The Product For The Following Diels-alder Reaction
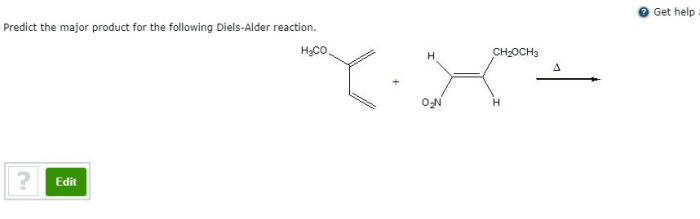
The Diels-Alder reaction is a cycloaddition reaction between a conjugated diene and a dienophile, resulting in the formation of a cyclohexene ring. The reaction proceeds through a concerted mechanism, involving the simultaneous formation of two new carbon-carbon bonds.
The reaction pathway can be illustrated as follows:
- The diene and dienophile approach each other in a parallel orientation, with the dienophile positioned above the diene.
- The π electrons of the diene and the π electrons of the dienophile interact to form a new σ bond between the two carbons that were originally part of the double bonds.
- Simultaneously, the π electrons of the diene and the π electrons of the dienophile interact to form a new σ bond between the two carbons that were originally part of the single bond in the diene.
- The result is the formation of a cyclohexene ring, with the two new carbon-carbon bonds formed in a cis configuration.
The stereochemistry of the product is determined by the orientation of the diene and dienophile in the transition state. If the diene and dienophile approach each other in an endo fashion, the product will have an endo stereochemistry. If the diene and dienophile approach each other in an exo fashion, the product will have an exo stereochemistry.
Predicting the Product
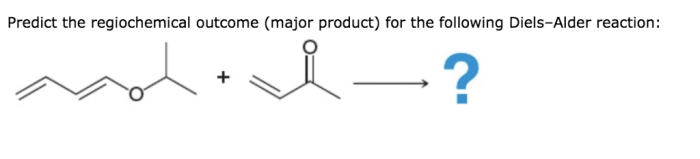
The product of a Diels-Alder reaction can be predicted using the following rules:
- The diene and dienophile must have a complementary relationship, meaning that the diene must have two double bonds and the dienophile must have one double bond.
- The diene and dienophile must approach each other in a parallel orientation, with the dienophile positioned above the diene.
- The product will be a cyclohexene ring, with the two new carbon-carbon bonds formed in a cis configuration.
- The stereochemistry of the product will be determined by the orientation of the diene and dienophile in the transition state.
For example, the reaction of 1,3-butadiene with maleic anhydride will produce a cyclohexene ring with an endo stereochemistry. This is because the diene and dienophile approach each other in an endo fashion, with the dienophile positioned above the diene.
Factors Affecting Product Formation
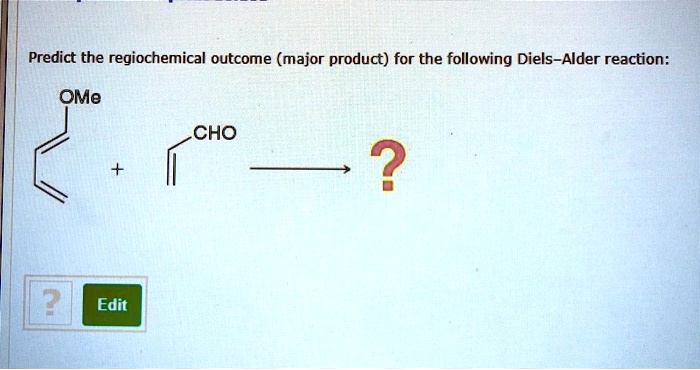
The formation of the Diels-Alder product is affected by a number of factors, including:
- Temperature:The Diels-Alder reaction is a thermally-driven reaction, and the rate of the reaction increases with increasing temperature.
- Solvent:The solvent can affect the rate and selectivity of the Diels-Alder reaction. Polar solvents, such as water, favor the formation of the endo product, while nonpolar solvents, such as benzene, favor the formation of the exo product.
- Substituents:Substituents on the diene and dienophile can affect the rate and selectivity of the Diels-Alder reaction. Electron-withdrawing substituents on the diene and electron-donating substituents on the dienophile favor the formation of the endo product, while electron-donating substituents on the diene and electron-withdrawing substituents on the dienophile favor the formation of the exo product.
By optimizing the reaction conditions, it is possible to control the formation of the Diels-Alder product.
Applications of the Diels-Alder Reaction

The Diels-Alder reaction is a powerful tool for the synthesis of cyclic compounds. The reaction has been used to prepare a wide variety of natural products and pharmaceuticals, including steroids, alkaloids, and antibiotics.
The Diels-Alder reaction can also be used to create complex molecular architectures. For example, the reaction can be used to prepare dendrimers, which are highly branched molecules with a well-defined structure.
Frequently Asked Questions
What is the Diels-Alder reaction?
The Diels-Alder reaction is a cycloaddition reaction between a conjugated diene and a dienophile, resulting in the formation of a cyclohexene ring.
How can I predict the product of a Diels-Alder reaction?
The product of a Diels-Alder reaction can be predicted using the endo rule, which states that the diene and dienophile will react in a way that minimizes steric hindrance.
What are the limitations of product prediction methods for the Diels-Alder reaction?
Product prediction methods for the Diels-Alder reaction are not always accurate, especially when the reaction is not under kinetic control.