Lewis dot structure and VSEPR worksheet introduces readers to the fundamental concepts of chemical bonding, providing a comprehensive understanding of the relationship between electron configurations and molecular geometry. This engaging resource offers a detailed exploration of Lewis dot structures, VSEPR theory, and their practical applications in chemistry.
The worksheet includes exercises and questions designed to reinforce the concepts discussed in the Artikel, making it an invaluable tool for students and educators alike.
Lewis Dot Structures: Lewis Dot Structure And Vsepr Worksheet
Lewis dot structures are a simplified representation of the valence electrons of an atom or molecule. They provide a way to visualize the electron configuration and bonding of atoms. In a Lewis dot structure, each element is represented by its chemical symbol, and the valence electrons are represented by dots placed around the symbol.
For example, the Lewis dot structure of hydrogen is H:, with one dot representing the single valence electron. The Lewis dot structure of oxygen is O:, with two dots representing the two valence electrons. The Lewis dot structure of nitrogen is N:, with three dots representing the three valence electrons.
The number of valence electrons in an atom is determined by its position in the periodic table. The elements in Group 1 have one valence electron, the elements in Group 2 have two valence electrons, and so on. The noble gases in Group 18 have a full valence shell and are unreactive.
VSEPR Theory
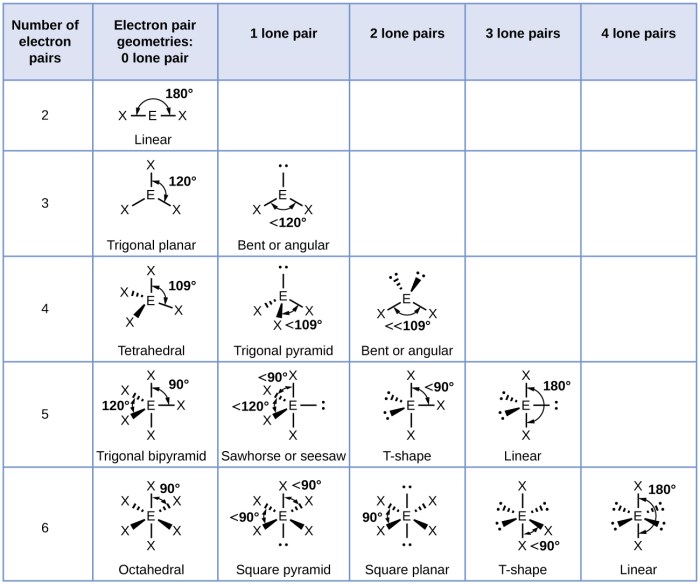
The VSEPR theory (Valence Shell Electron Pair Repulsion theory) is a model that predicts the geometry of molecules based on the number of valence electron pairs around the central atom.
The VSEPR theory states that the electron pairs around the central atom will arrange themselves in a way that minimizes the repulsion between them. This means that the electron pairs will be as far apart as possible.
The VSEPR theory can be used to predict the geometry of a molecule by counting the number of valence electron pairs around the central atom. The following table shows the relationship between the number of valence electron pairs and the molecular geometry:
| Number of Valence Electron Pairs | Molecular Geometry |
|---|---|
| 2 | Linear |
| 3 | Trigonal planar |
| 4 | Tetrahedral |
| 5 | Trigonal bipyramidal |
| 6 | Octahedral |
Worksheet Activities
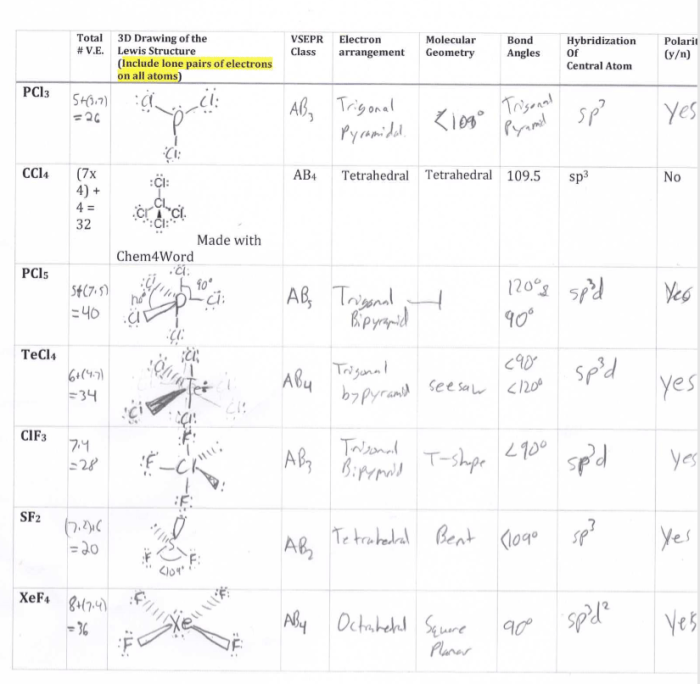
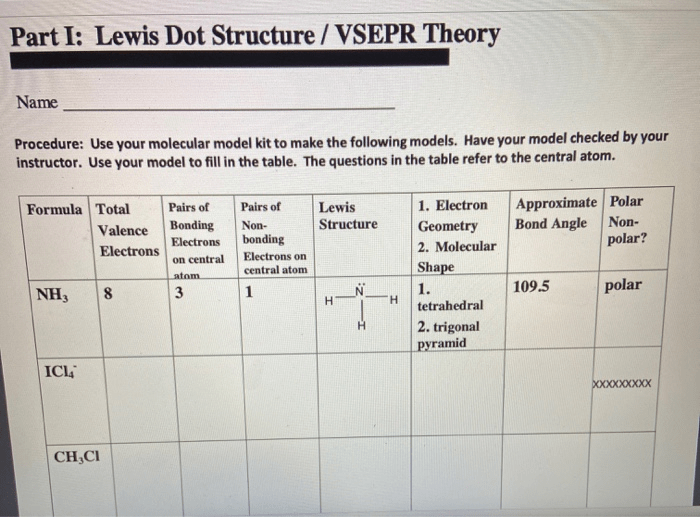
Worksheet activities can be used to help students learn about Lewis dot structures and VSEPR theory. These activities can include exercises on drawing Lewis dot structures, predicting molecular geometry using VSEPR theory, and combining Lewis dot structures and VSEPR theory to solve problems.
One example of a worksheet activity is to have students draw the Lewis dot structure of a given molecule and then predict its molecular geometry using VSEPR theory. Another example is to have students solve a problem that requires them to use both Lewis dot structures and VSEPR theory, such as predicting the molecular geometry of a molecule that has a lone pair of electrons.
Applications
Lewis dot structures and VSEPR theory are used in a variety of applications in chemistry. These applications include predicting molecular properties, such as bond length and bond angle, and predicting molecular reactivity.
For example, Lewis dot structures can be used to predict the polarity of a molecule. A polar molecule is a molecule that has a separation of charge, with one end of the molecule being positive and the other end being negative.
The polarity of a molecule can be predicted by looking at the Lewis dot structure of the molecule. If the molecule has a lone pair of electrons, then the molecule will be polar.
VSEPR theory can be used to predict the reactivity of a molecule. A reactive molecule is a molecule that is likely to undergo a chemical reaction. The reactivity of a molecule can be predicted by looking at the VSEPR model of the molecule.
If the molecule has a lot of steric hindrance, then the molecule will be less reactive.
Resources, Lewis dot structure and vsepr worksheet
- Lewis Dot Structuresby LibreTexts
- VSEPR Theoryby Khan Academy
- Worksheet on Lewis Dot Structures and VSEPR Theoryby Chemistry LibreTexts
FAQ Guide
What are Lewis dot structures?
Lewis dot structures are diagrams that represent the valence electrons of atoms and molecules. They show the arrangement of electrons in the outermost energy level of an atom or molecule.
What is VSEPR theory?
VSEPR theory (Valence Shell Electron Pair Repulsion theory) is a model that predicts the geometry of molecules based on the number of valence electron pairs around the central atom.
How are Lewis dot structures and VSEPR theory related?
Lewis dot structures provide information about the number of valence electrons around an atom, which is essential for applying VSEPR theory to predict molecular geometry.