Unit atomic structure ions – ws #3 invites us on an enlightening journey into the realm of atomic particles, ions, unit cells, and crystal structures. This comprehensive guide delves into the fundamental building blocks of matter, their interactions, and their profound impact on the properties of materials.
From the intricate arrangement of protons, neutrons, and electrons within atoms to the formation and behavior of ions, we unravel the secrets that govern the behavior of matter at the atomic level. The concept of unit cells and crystal structures provides a deeper understanding of the organization and properties of crystalline materials, paving the way for advancements in fields ranging from materials science to pharmaceuticals.
Atomic Structure

An atom is the smallest unit of matter that retains all the chemical properties of an element. It is composed of a dense central nucleus surrounded by a cloud of electrons. The nucleus contains protons and neutrons, while the electrons orbit the nucleus in discrete energy levels.
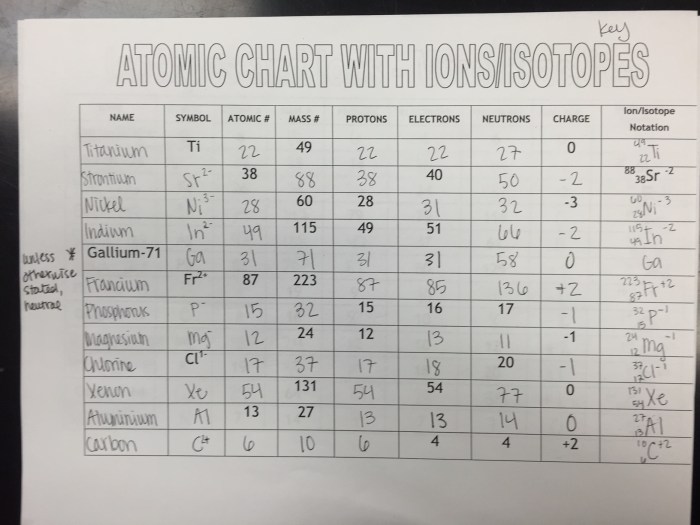
Protons are positively charged particles, while neutrons are neutral. The number of protons in an atom determines its atomic number, which identifies the element. The number of neutrons can vary, giving rise to isotopes of the same element.
Electrons are negatively charged particles that orbit the nucleus in specific energy levels. The lowest energy level is closest to the nucleus, and the energy levels increase as you move away from the nucleus.
Relationship between Protons, Neutrons, and Electrons, Unit atomic structure ions – ws #3
The number of protons and electrons in an atom must be equal for the atom to be electrically neutral. If the number of protons and electrons is not equal, the atom will have a net charge and is called an ion.
Ions
Ions are atoms or molecules that have lost or gained electrons, resulting in a net electrical charge. Ions can be positively charged (cations) or negatively charged (anions).
Cations are formed when an atom loses one or more electrons, while anions are formed when an atom gains one or more electrons.
Properties of Ions
Ions have different properties than neutral atoms. For example, ions are attracted to oppositely charged ions and can form ionic bonds. Ions can also conduct electricity in solution.
Unit Cell: Unit Atomic Structure Ions – Ws #3
A unit cell is the smallest repeating unit of a crystal structure. It is a three-dimensional arrangement of atoms or molecules that represents the entire crystal.
There are different types of unit cells, including primitive, body-centered, and face-centered. The type of unit cell determines the symmetry and properties of the crystal.
Relationship between Unit Cell and Crystal Structure
The unit cell is the building block of a crystal. The arrangement of unit cells in space determines the crystal structure. There are different types of crystal structures, including cubic, tetragonal, orthorhombic, and hexagonal.
Crystal Structure
Crystal structure refers to the arrangement of atoms or molecules in a solid material. Crystals are characterized by their regular and repeating patterns.
The crystal structure of a material determines its physical properties, such as its hardness, strength, and electrical conductivity.
Types of Crystal Structures
There are many different types of crystal structures, including cubic, tetragonal, orthorhombic, and hexagonal. Each type of crystal structure has a unique arrangement of atoms or molecules.
Expert Answers
What is the significance of the number of protons, neutrons, and electrons in an atom?
The number of protons determines the element’s identity, while the number of neutrons affects its isotopic composition. The number of electrons influences the atom’s chemical properties and its ability to form ions.
How do ions differ from neutral atoms?
Ions have a net electrical charge due to the gain or loss of electrons, while neutral atoms have no net charge.
What is the relationship between the unit cell and the crystal structure of a material?
The unit cell is the smallest repeating unit of a crystal structure, and its arrangement determines the overall symmetry and properties of the crystal.